Forms of a Meso Compound
The term “meso compound” is used to describe a type of organic compound. This class of compounds is structurally similar to a chiral compound, but have different chemical and physical properties. Enantiomers and Diastereomers both rotate light in the same direction, but they have different potential energies. In addition, they both have multiple chiral centers. In this article, we’ll discuss how the different forms of this molecule are classified.
Diastereomers have different physical and chemical properties
Diastereomers differ from enantiomers in their chiral centers and are not superimposable. In addition, their chiral centers are assigned differently. Diastereomers are not identical to each other in all aspects, such as melting and boiling points. The difference in these two properties is due to their different stereocenter arrangements. Here is a brief overview of the different types of stereoisomers.
Despite their similarity in molecular constitution, diastereomers differ in their three-dimensional spatial arrangements. This makes them useful in chiral synthesis. Separation of diastereomers is achieved by chromatography, recrystallization, or ketonization. Diastereomers of a meso compound can also be isolated through stereochemistry.
Enantiomers and diastereomers are mirror images of each other, but their structures are not identical. Diastereomers differ in their chiral centers but are not superimposable. They cannot overlap each other. Diastereomers are also different in terms of their enantiomeric properties. Diastereomers are classified into two types, stereoisomers and enantiomers.
Enantiomers and stereoisomers are identical, but diastereomers have different chemical and physical properties. The diastereomers have different melting and boiling points, different Gibbs free energies, and differ in optical activity. Diastereomers are optically active and can be separated by fractional crystallization, chromatography, and other processes.
Cyclic-1,2-diols react with periodic acids, such as lead tetraacetate. Diastereomers have higher reaction rates than trans isomers, but the trans isomer may not undergo this reaction at all. The type of functional groups present in diastereomers is another important factor in the determination of the physical and chemical properties of meso compounds.
A meso compound can be either chiral or meso. A meso compound can have as many as two stereoisomers, but it is possible for a meso compound to have less than two. Diastereomers, for example, have different polarized light bend. One will bend light clockwise and the other will bend it counterclockwise.
A meso compound can be divided into two enantiomers: the R and the S enantiomer. Diastereomers are mirror images of one another. Diastereomers are chemically distinguishable because they have different atoms bonded to the carbon center. They are also mirror images of each other. They are also not superimposable.
Enantiomers have equal potential energies
The two enantiomers of a meso compound have equal potential energies. The racemic mixture has a 50:50 mixture of both R and S. Generally, chiral compounds have identical b.p.’s, molecular weights, and boiling and melting points. The difference in their potential energies is represented by ee, and these enantiomers have equal reactivity at various temperatures.
Molecular structures in meso compounds have chiral centers, but the stereocenters are not identical. The stereochemistry of the two halves of the compound should cancel out, and the polarimeter will not show (+) or (-) signs. These molecules have equal potential energies, but they have different degrees of solubility. These properties will affect the rate at which the two enantiomers react with each other.
The chiral nature of meso compounds allows scientists to understand the differences between them. By studying the chiral structure, it is possible to understand how enantiomers interact with biological receptors. This is important for drug development, as one of these compounds can be highly effective in treating diseases and other ailments. There are numerous examples of pharmaceuticals that act by binding to receptors in the body. These receptors have a chemical specificity that helps them attach to their target sites.
As the number of substituents increases, the stereogenic center changes. The stereogenic center may be carbon or a quaternary nitrogen atom with four different groups attached. Hence, the stereogenic center changes with respect to the atomic number, and the two meso compounds have different enantiomers. The convention used for enantiomeric identification is the (R,S) system. The R enantiomer is referred to as the “R” enantiomer, and the other one is known as the “S” enantiomer.
The Fischer projection formula is helpful for understanding isomer configurations. It is helpful to visualize the two meso-compound formulas as three-dimensional structures. These three-dimensional models can be rotated 180o in the plane. The same goes for the diastereomer of a meso compound. Using this method, we can distinguish between the two identical molecules.
Diastereomers rotate light exactly opposite to each other
As the name suggests, diastereomers are optical compounds with different rotational properties. These compounds differ from each other by their placement of substituted groups around the atom. They also differ in the chemical reactivity and physical properties of the chiral centers, and are named as enantiomers or diastereomers. Each one exhibits a slightly different rotational properties.
Enantiomers and diastereomers are mirror images of each other but their optical properties are different. In the table below, we can see the differences and similarities of these two types of molecules. Diastereomers have the same chemical and physical properties, but rotate light in opposite directions. Therefore, these two kinds of molecules are classified as diastereomers. There are several types of chiral centers in diastereomers.
In chemistry, diastereomers are different from enantiomers in two ways: one has a single chiral center while the other has two. Each is the same but has an opposite chirality center. The two are not mirror images of each other, but rather opposite versions of each molecule. The only difference between one and the other is in the chirality center.
The optical rotations of enantiomers depend on several factors. Solvents, solvent, and environmental conditions influence the rotations. Therefore, it may not be possible to distinguish ‘(+)’ from ‘(-)’ enantiomers. This is because ‘(-)’ enantiomers rotate light in the opposite direction, while ‘(-)’ enantiemers rotate light exactly opposite each other.
Benzene is a typical example of an enantiomer, and its enantiomer is the levorotatory isomer. The levorotatory isomer has a rotation of -3.12. The Latin terms “sinister” and’rectus” were used to distinguish the two. Molecular substituents are ordered in stereocenters by decreasing atomic number. For example, the substitutes in 2-bromobutane are Br, CH3-C, thereby creating the stereocenters.
In the optical world, this effect is explained by the enantiomers’ rotation of the plane of polarization. This rotation is called chirality, and chiral substances rotate plane-polarized light. Molecular chirality allows us to distinguish pure enantiomers from their corresponding enantiomers. Moreover, chiral compounds are optically active, and this property is called a stereoisomer.
Diastereomers have multiple chiral centers
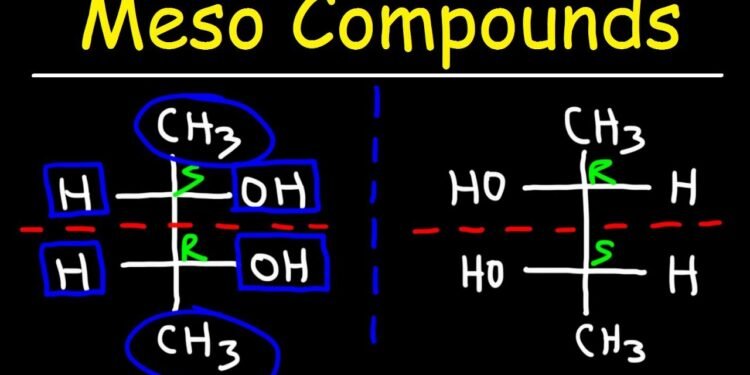
There are two main types of diastereomers of meso compounds. The left type consists of compounds with two R chiral centers and the right type consists of compounds with two S chiral centers. Diastereomers differ in the arrangements of chiral centers at the double bonds and rings. This fact is important because these two types of compounds differ in many other properties, including their melting and boiling points.
The chiral centers of two different meso compounds make it difficult to make them interchangeable, but it is possible to synthesize two or three-component molecules that have a single chiral center. Another example of a meso compound is tartaric acid. The chiral center of tartaric acid results in three stereoisomeric forms. Louis Pasteur studied the subtle stereochemistry of tartaric acid salts, resulting in the discovery of the meso compound class.
Another type of diastereomers is aldopentose compounds. The structure of aldopentose is diastereomeric, and it is possible to have two chiral centers in one of these structures. For instance, ribose and arabinose are epimers at C-2 and C-3, while xylose and ribose are not epimers.
The difference between diastereomers and enantiomers is the configuration of the stereocenter. Diastereomers are mirror images of one another. Diastereomers of meso compounds are not superimposable. However, they are similar chemically, and can have similar properties. It is also possible to create new compounds by combining several diastereomers of the same compound.
A diastereomer of a meso compound has two chiral centers and is the mirror image of the original. The trans stereoisomer does not have a plane of symmetry, which means that its mirror image is different from the original. Therefore, it is important for organic chemists to identify planes of symmetry in molecules. This is essential for understanding how chiral centers are arranged in a molecule.
Diastereomers of meso-tartaric acid exhibit a similar configuration of chiral centers. As a result, the chiral center of the meso-tartaric acid is oriented parallel to the plane of symmetry of the meso-tartaric molecule. The third conformer is chiral in nature. However, it still retains its achiral shape.